Sleep can be defined in various ways:
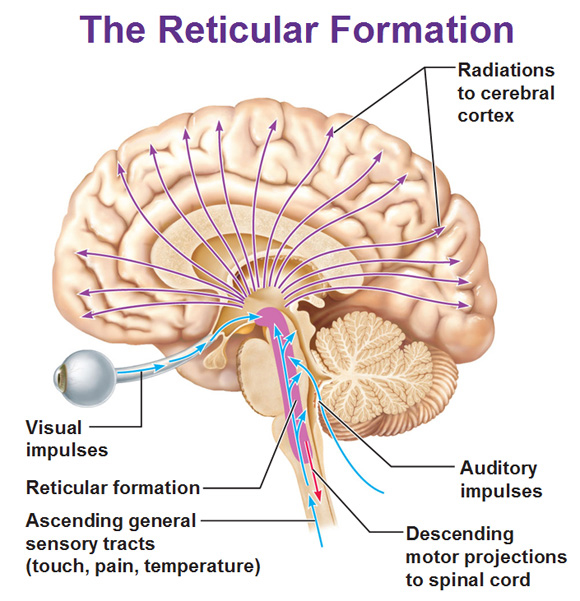
Sleep occupies about a third of our lives, and sleep deprivation causes major psychological and behavioural changes. Sleep is not a simple reduction/cessation of brain activity but an active state, characterised by a sequence of precisely controlled patterns of cortical activity. All parts of the cortex are connected to the thalamus and other nuclei in the brainstem or forebrain. Cortical activity is controlled by the hypothalamus and the ascending reticular formation, and there are nuclei there that project to and influence the activity of all parts of the brain. These neurones use acetylcholine, noradrenaline, histamine and serotonin, and peptides such as galanin or the orexins as neurotransmitters. |
|
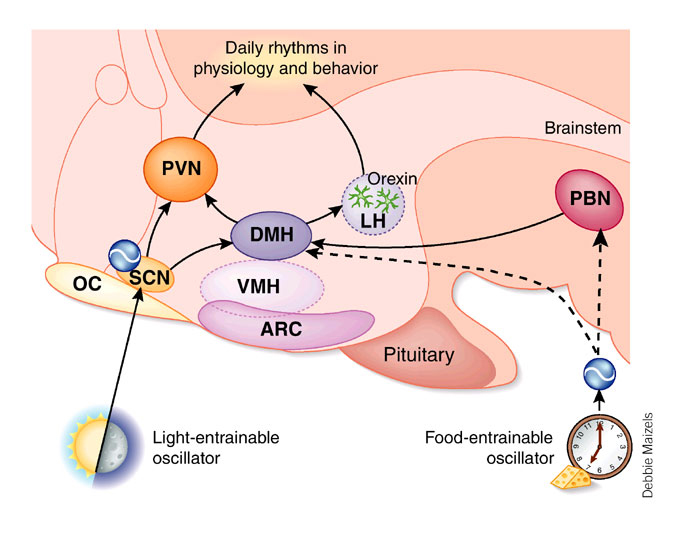
Hypothalamic Nuclei involved in Sleep/Waking Cycle Although not really part of the brainstem reticular formation, several hypothalmic nuclei are involved, along with the brainstem, in the sleep waking cycle. The suprachiasmatic nucleus determines the rhythm of sleeping and waking, and the lateral hypothalamic nuclei that contain orexins have recently been found to be of importance in the urge to sleep. Some hypothalamic nuclei influence the activity of brainstem pathways involved in arousal (waking). |
|
|
Sleep is a daily event, entrained by light, food intake and other rhythms. The suprachiasmatic nucleus (SCN, in the hypothalamus) appears to be essential for sleep rhythms: i.e. it appears to be (in animals) the primary ‘biological clock’ for sleep and receives visual inputs which can provide information about the light-dark cycle. Many bodily functions have diurnal rhythms - these functions are determined by the 24 hour cycle of day and night - light and dark, and these visual cues to time keep neurones concerned with sleep timed to a 24 hour clock. Hormonal secretions (e.g. the release of cortisol by the adrenal cortex) and sleep are just two of many examples of bodily functions with a dirunal rhythm. In the absence of the daily 24-hour cycle of light and dark, the biological circadian rhythm can move to a different cycle (other than 24 hours). For example, when a subject was isolated, living in an underground bunker, his natural rhythm changed from a 24 hour cycle to a 26 hour cycle, and he returned to normal when given the normal light-dark cues. Eating is another cyclic process and the 'food-entrainable oscillator' referred to opposite indicates that food intake also influences the hypothalamus. People tend to sleep at night and in the afternoon (after lunch!). |
* PVN=ParaVentricular Nucleus; DMH=Dorso-Medial Hypothalamus; LH=Lateral Hypothalamus; ARC= Arcuate Nucleus; OC= Optic Chiasma; PBN=Parabrachial Nucleus |
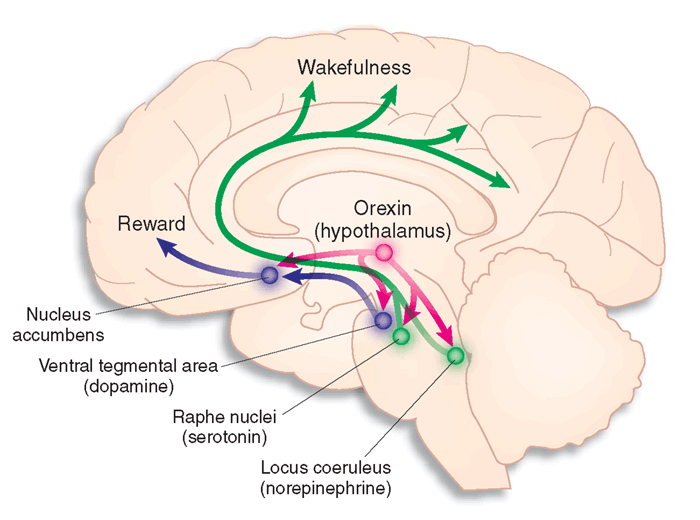
The Orexins (Orexins A and B) are two excitatory neuropeptide transmitters produced from the same gene by groups of neurones in the lateral and posterior hypothalamus. The axons of orexin neurones project to the cerebral cortex and brainstem nuclei, including the locus coeruleus, the raphe nucei and the ventral tegmental area. Orexin neurons excite dopaminergic, noradrenergic and cholinergic neurones and stimulate food intake, wakefulness and energy metabolism. They are also involved in arousal, and deficiency of orexins leads to the sleep disorder narcolepsy. The diagram opposite shows that orexins influence the activity of many ascending pathways. The role of orexins in exciting the Nucleus accumbens is interesting in that this nucleus is involved in 'feeling good' and may be involved in the mechanism of action of addictive drugs and alcohol. |
|
Nuclei of the Reticular Formation involved in Sleep/Waking Cycle |
|
The ascending reticular formation originates in the brainstem (opposite), and projects to the hypothalamus, limbic system and all areas of the cortex. In fact there are three or more groups of neurones within the Ascending Reticular Formation, involved in the sleep/waking cycle. Each of the following groups of neurones becomes inactive at the start of normal sleep:
Sleep is an active process, thought to be intitated in the anterior hypothalamus, and the stages of sleep are programmed in a relatively predictable sequence, and appear to be controlled by different, but linked, neurochemical systems. Neural Mechanisms of Sleep Sleep: In experimental animals, sleep can be initiated actively by electrical stimulation of the preoptic area of the hypothalamus, particularly the suprachiasmatic nucleus. Waking: In addition to neurones that induce sleep, the rostral reticular formation at the junction of the upper pons and midbrain contains a population of neurones that are required for wakefulness; these form part of the ascending reticular activating system and these neurones include cholinergic and noradrenergic pathways. |
* |
|
|
|
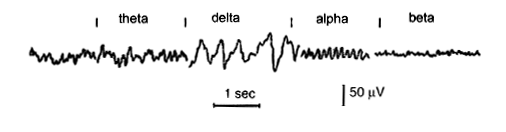
The Electroencephalogram (EEG) One channel of the Electroencephalogram consists of a recording of the potential difference between two points on the surface of the scalp, which depends on the electrical activity of the underlying cerebral cortex. Clinical EEG recordings are usually made using many electrodes, placed in standard positions in the scalp, established by an International Committee (Jasper, 1958). The diagram oposite shows these standard positions and the associated nomenclature. Each electrode pair samples the potentials produced by the underlying cortex, so the spatial distribution of waves - arising from the frontal, temporal or occipital cortex - can also be analysed. EEG: brain Waves A channel in the EEG measures the voltage between two points on the scalp, and these potentials can be described in terms of their frequency (number of cycles per second) and amplitude (microvolts). The normal waves are:
 * *
|
The rhythm of the EEG is believed to depend on oscillatory waves of electrical activity in thalamo-cortical circuits that can be modulated by cholinergic, noradrenergic and serotoninergic pathways, such as those originating in the ascending reticular formation of the brainstem. In an awake resting subject, the resting rhythm is the alpha rhythm. Concentration, thinking, doing mental arithmetic, etc, cause the EEG to adopt a faster Beta rhythm. Sleep is associated with slower EEG rhythms, such as the Delta rhythm. Patients in a deep coma show even lower frequencies in their EEGs. |
The normal rhythm is the alpha rhythm, and consists of large amplitude waves when the eyes are closed. When the eyes are closed an alpha rhythm can be recorded over the occiptal cortex (the visual receiving area). But when the eyes are open the rhythm becomes faster because of changing patterns of light falling on the retina.
|
In other ares of the cortex, the alpha rhythm at rest changes when the subject thinks or does mental arithmetic. the amplitude gets smaller and the waves of electrical activity become more frequent: this is the beta rhythm. Beta waves are observed in all age groups, are small in amplitude. Certain drugs, such as benzodiazepines, increase the frequency of beta waves. Theta and Delta rhythms are present during sleep, and the deeper the sleep the slower and larger are the waves. These rhythms are due to the degree of synchronisation of activity within the cortical networks beneath the electrodes. If neurones become active together the combined effect to a larger wave of electrical activity; if many neuones are active at different times, the waves are more frequent, and the amplitude is smaller. So the degree of synchronisation of activity in these networks determines whether the EEG shows large amplitude slow waves or low amplitude fast waves. In the diagram opposite note the effects of opening and closing the eyes on the EEG rhythm recorded from the occipital region in an awake individual.
|
|
|
|
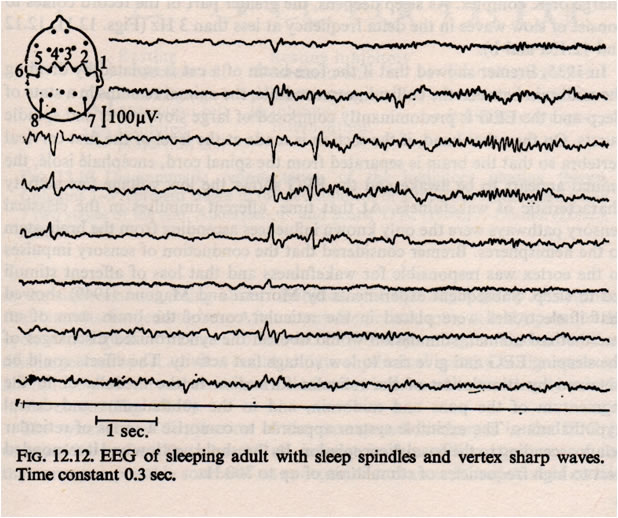
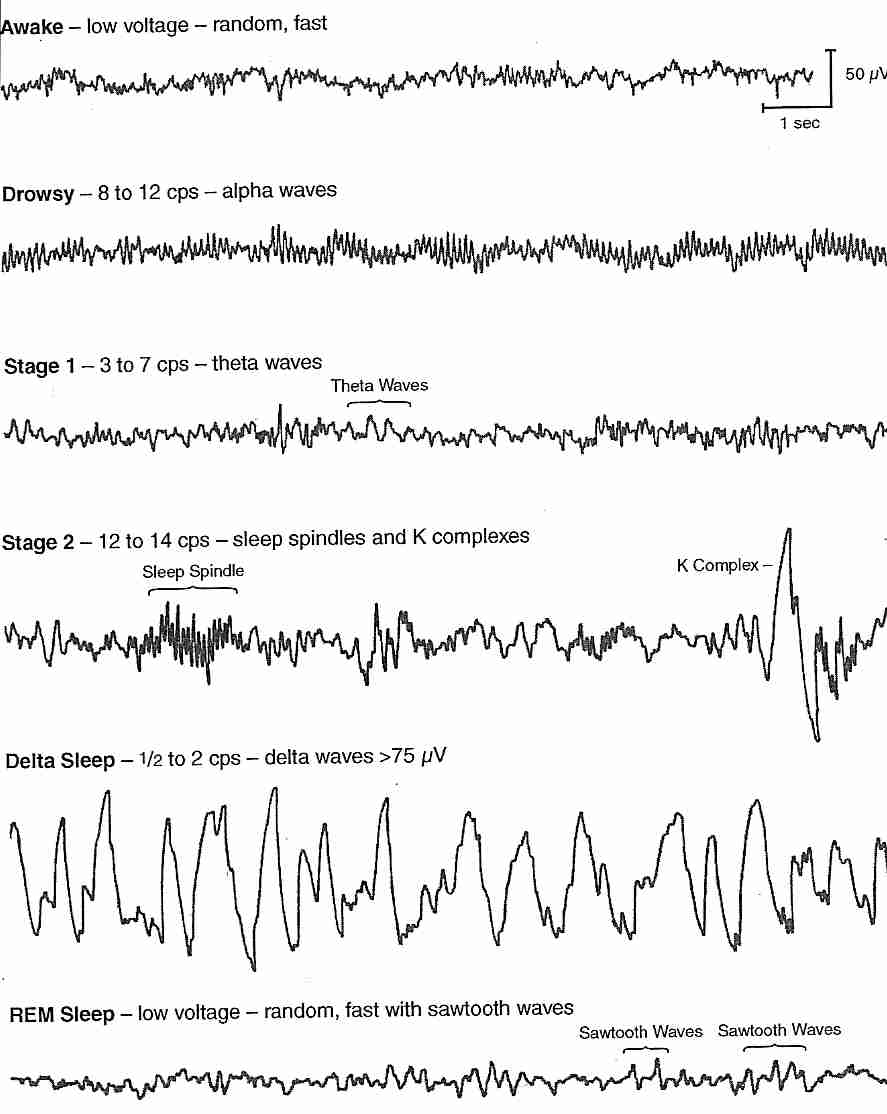
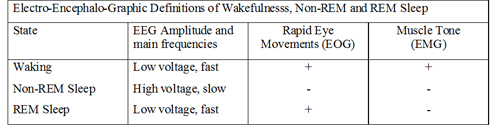
During normal sleep there are changes in the amplitude and frequency of the EEG. Slow wave sleep appears to be associated with a neurological ‘resting state’. As the EEG waves become slower, they also increase in amplitude; this is interpreted as meaning that the cortical neurones start to be active together, and their activity is said to be synchronised. Synchronization means the appearance of large slow waves in the EEG: this is associated with the sleeping state. Arousal or Waking is associated with desynchronisation. Desynchronization is the appearance of small high frequency waves. During normal deep sleep, the EEG shows high voltage slow waves, and the muscle tone is reduced, and rapid eye movements are absent. The eight EEG traces opposite are recordings made from the central region of the cortex (electrodes 1-6) and from the occipital cortex (7 and 8). Sleep spindles are a normal EEG pattern and are clearest towards the right end of traces 3 and 4. Several channels also show vertex sharp waves towards the middle of the recording. | EEG Example
|
Slow Wave Sleep The frequency of the EEG slows and the amplitude of the brain waves increases as the depth of sleep increases. Stage 1 (drowsy) proceeds to Stage 2 (light sleep), which is characterised by slower, larger amplitude waves, which are interrupted by intermittent spike clusters called ‘sleep spindles’. Sleep spindles are periodic bursts of high frequency activity that last 1-2 seconds, and arise because of interactions between thalamic and cortical neurones. ‘K-complexes’ = a delta wave + a spindle -are also present in Stage 2 sleep. In Stage 3 sleep the number of spindles diminishes, and in Stage 4, the sleep is very deep and accompanied by delta waves (frequencies of <4 Hz). Normally the progression between waking and stage 4 sleep takes about an hour. During Stage 4 sleep the oxygen consumption of the brain is reduced by up to about 45%, and body temperature drops. Deprivation of slow wave sleep leads to a breakdown of homeostatic functions and eventually, in experimental animals, to death. |
|
|
A second type of EEG pattern during sleep was identified in 1953 and given the name Rapid Eye Movement Sleep (REM Sleep); it is sometimes called 'Paradoxical Sleep'. During REM sleep EEG activity is increased to a level characteristic of the waking state, and is associated with dreaming. REM sleep is associated with changes in activity in cholinergic and noradrenergic systems arising from the brainstem reticular formation.
|
The EEG changes of REM sleep are similar to the waking state, the oxygen consumption is similar to normal, but the tone of the body musculature is low, and muscular movements are absent. |
|
|
|
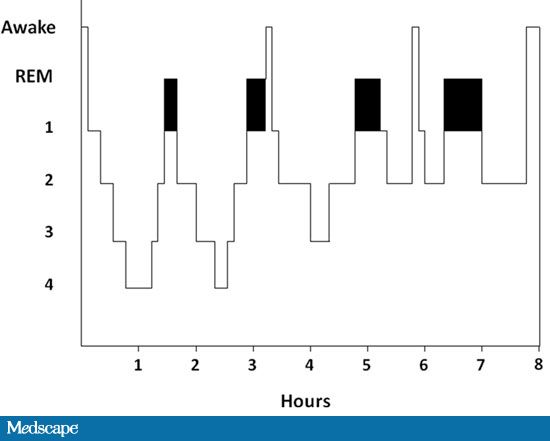
Sleep Architecture Sleep architecture refers to an analysis of the different stages of EEG sleep - i.e. the proportions of different EEG stages of sleep - during a night's sleep.
|
|
Sleep and Dreaming If a person is asleep and is awakened during an episode of REM sleep, they recall dreaming. 85%+ of subjects dream during REM sleep, whereas less that half those wakened during stage 4 recall dreaming, and if they do recall a dream the events of the dream are less vivid. The ability to recall a dream is lost within 8 minutes of starting Stage 4 sleep. Eye movements appear to be related to dream imagery. Eventful dreams have more REMs. Autonomic Events in REM sleep:
| Emotional content of dreams: In one study, 60% of dreams were associated with sadness, apprehension or anger. 18% were happy or exciting. Only 1% associated with sexual feelings or acts, even though erection is a common finding during REM sleep. Sensory events are reported during REM sleep: these include visual, auditory and vestibular sensations. These may be described as though the individual is ‘floating, flying, falling, or soaring’. REM sleep is accompanied by hypotonia - a generalised loss of EMG activity throughout the body. So it is maube surpriseing that movement is commonly reported by subjects awakened during a dream. The limbic system is known to be activated during REM sleep, and some workers believe that REM sleep is off-line processing of information that has been recently acquired. |
|
|
|
Effects of Sleep Deprivation Stage 4 slow wave sleep and REM sleep behave differently following a period of sleep deprivation: Deprivation of Slow Wave Sleep
Deprivation of REM sleep There are various methods of curtailing REM sleep without interference with stage 4 sleep and it is clear that there can be a major deprivation of REM sleep without gross behavioural changes. However there may be an increased anxiety and irritability. | Effects of Drugs on Sleep Architecture Hypnotic drugs generally cause:
This raises the question as to whether hypnotics actually produce a ‘good’ deep sleep. Deprivation of REM sleep causes a rebound effect - an excessive increase in REM sleep after the period of deprivation. Despite the reduction in slow wave sleep, hypnotics do not produce changes in the secretion of adrenal or pituitary hormones.
|